Video Views and Reviews
For this column I return to the Supplemental Material list of the Journal of Cell Biology (JCB), which I last examined for Winter 2002 and which is readily accessible by subscribers and nonsubscribers alike at http://www.jcb.org/supplemental. Generally speaking, the research videos published in JCB can be appreciated as stand-alone records, which is fortunate because the articles themselves can be accessed on-line only by subscribers. The journal format provides each video with its own caption, in addition to any contextual references. A separate descriptive section—designated Online Supplemental Material (OSM)—is located at the end of each article, which summarizes the caption detail. Unfortunately, however, the “Supplemental Material” entry provides no indication whether any given supplement contains video records (and, if so, how many) or such ancillary material as still figures, additional tables, or protocol detail. Moreover, the citations are arranged more or less by year (in reverse chronological order), but they are not delineated further within any given year, by volume, issue, or first author.
Thus, if you enjoy browsing in used book stores as I do, you will enjoy scanning the Supplemental Material listing for interesting material, and as in any good used book store you are sure to be rewarded with a find or two! I provide URLs for the videos that accompany each of the articles I review below, for the more focused reader and viewer.
Again, I invite your comments on these reviews and your suggestions of other peer-reviewed videos for possible review as educational material [[email protected]].
CALCIUM SIGNALING AND PHAGOCYTOSIS IN NEUTROPHILS
Neutrophils are white blood cells specialized for scavenging cellular debris and consuming infectious microorganisms in a process called phagocytosis. What seems to make neutrophils especially voracious is the presence of antibodies (or opsonins) coating the surface of foreign cells. Recently, Dewitt and Hallett (2002) examined the role of cytoplasmic calcium in mediating neutrophil phagocytosis of opsonin-coated particles, using neutrophils that had been loaded with a compound (Fura-2) that changes its fluorescence properties when it binds with calcium. Their observations are accompanied by two videos, the first recording changes in calcium concentrations as an opsonized particle is phagocytosed (Figure 1) and the second exhibiting similar changes when a particle is touched to the surface of a neutrofil and then withdrawn (Figure 2).
The changes in Fura fluorescence are quite dramatic, as Figure 1 illustrates, especially the wave of calcium that spreads throughout the neutrofil following phagocytosis. The video documents this wave for an introductory audience. Unfortunately, the initiation of the wave and the early stages of phagocytosis are not temporally well resolved, however, due to the time-lapse nature of the process required to obtain reliable Fura emission ratio measurements and to produce pseudocolored images that accurately reflect cytoplasmic calcium levels. It is therefore difficult to distinguish the rapid sequence of events associated with particle binding, and many observant students will likely question whether a localized increment in calcium precedes (and causes), follows (and is the result of), or merely accompanies (and possibly is only correlated with) phagocytosis. To clarify this important issue, the authors treated neutorphils with the microfilament inhibitor, cytochalasin, which inhibits phagocytosis and the rapid changes in cell shape that accompany this process. The drug had no effect on the calcium wave, however, consistent with calcium triggering the phagocytic response to opsonin binding at the neutrophil surface (rather than being a secondary response to phagocytosis).
Opsonized particles trigger phagocytosis through their binding with β2 integrin, an integral membrane protein of the neutrophil plasma membrane. The authors document the nondiffusible nature of opson–integrin interaction by touching a pipette coated with opsin to a neutrophil, in an impressive set of three video movies obtained simultaneously from a single experiment, as illustrated in Figure 2.
Advanced students may want to explore additional data in the paper concerning the possible intermediacy of calpain, a calcium-stimulated protease, in activating these integrins and triggering phagocytosis. They might also enjoy speculating how the phagocytosis occurred in 3 instances (of a total of 36) where no changes in calcium could be detected and presumably integrin binding was absent. Unfortunately, no video records of these additional experiments are provided.
ROLE OF CALCIUM SIGNALING IN PLATELET AGGREGATION
Calcium signaling is ubiquitous, it seems, and one of the earliest signaling roles documented for this divalent ion is its part in triggering platelet aggregation and blood clotting. Nesbitt et al. (2003) have nicely modeled this process by flowing platelets over a surface covered with fibrillar collagen (Type I or III) and von Willebrand factor (vWf), both of which promote clotting. They prelabeled the platelets with calcium-sensitive, fluorescing dyes (Oregon green linked with BAPTA-1 and Fura red) and then observed the change in fluorescence with confocal microscopy as the platelets became tethered to the substratum and/or linked with each other in the flow chamber to form thromboses. These events are well documented in a strikingly colored video, as depicted in Figure 3.
Figure 1. Phagocytosis of an opsonized particle (white sphere) by a neutrophil loaded with the calcium fluorochrome, Fura-2. Changes in the fluorescence ratio of Fura excited at two wavelengths is due to increasing cytoplasmic calcium concentrations and is reflected by changes in pseudocoloring from blue (ca. 100 nM) to green (ca. 600 nM). http://www.jcb.org/cgi/content/full/jcb.200206089/DC1/1. Reproduced from The Journal of Cell Biology, 2002, vol. 159, pp. 181–189, by copyright permission of The Rockefeller University Press.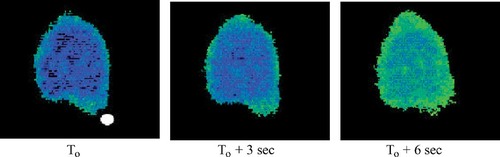
Introductory students will quickly appreciate the colorful bursts of intracellular calcium that accompany platelet aggregation (evident as episodes T1 and T2 in the video), and the more observant among them will also appreciate the periodic calcium “flashes” (or oscillations) that appear in individually tethered platelets (“S” in Figure 3a). The video would benefit from close scrutiny and repeated viewing, however, because not all platelet aggregates exhibit calcium flashes during the time course of the video. Moreover, intermediate students and their teachers may find the video sequence a good starting point for discussing the source of the calcium in these flashes and how the calcium levels can rise and fall in an oscillatory manner. Indeed, they might find the authors' use of “intercellular calcium communication” (ICC) in the title and elsewhere in the text somewhat ambiguous: Does ICC refer to a direct flow of calcium among contacting platelets, as the term would apply to cells in an epithelium, or to a cascade of calcium elevations that is mediated by surface contacts among aggregating platelets. The Nesbitt et al. paper describes a series of experiments that investigate these questions and the intermediacy of the purinergic ADP receptor. A journal club session might well be devoted to examining the data in some detail.
Figure 2. Tryptich images of the time course of the rise in cytoplasmic calcium concentration (a) in the fluorescent image of a neutrophil (b) being touched with an opsonized particle tethered to a micropipette (c). http://www.jcb.org/cgi/content/full/jcb.200206089/DC1/2. Reproduced from The Journal of Cell Biology, 2002, vol. 159, pp. 181–189, by copyright permission of The Rockefeller University Press.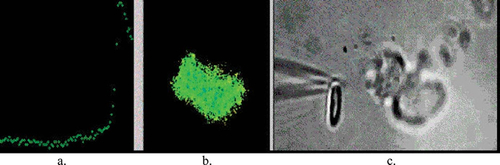
ACTIN POLYMERIZATION, PARTICLE STREAMING AND LAMELLIPODIAL LOCOMOTION
Some of us have a difficult time appreciating how the assembly of cytoskeletal subunits can generate a lamellipodial form of cellular motility or the intracellular movement of such pathogens as Shigella or Listeria (see Alberts et al., 2002, p. 1446 ff). In part, this difficulty stems from our failure to appreciate that this form of locomotion occurs much more slowly than a naive viewing of time-lapse images would indicate. Also, the process has no ready mechanistic counterpart in our everyday experience.
I was delighted, therefore, to see the recent investigation by Wiesner et al. (2003) of an in vitro model of particle motility using polystyrene microspheres and the controlled assembly of monomeric actin (G-actin). Briefly, the authors recorded the behavior of particles coated with the cell-signaling protein, N-WASP (the activator of the Arp complex) in a defined medium containing ATP, various cytoplasmic ions, and various purified proteins thought to be necessary and sufficient for actin polymerization in situ: fibrous actin (F-Actin), profilin, gelsolin, Arp 2/3 complex, and ADF (actin-depolymerizing factor). Three phase contrast stills abstracted from the video are presented in Figure 4, which depict the relative movements of several beads and their accompanying actin “comet tails.”
Figure 3. Platelets exhibiting low intracellular levels of calcium appear blue, and as their calcium levels increase, their color changes from red to yellow to white. A region—T1—is depicted where aggregation apparently triggers simultaneous elevations in calcium when platelets make contact. The platelets in this field are flowing upward at a speed magnified about sixfold by time-lapse recording; a and b were recorded about 4 sec apart. http://www.jcb.org/cgi/content/full/jcb.200207119/DC1/1. Reproduced from The Journal of Cell Biology, 2003, vol. 160, pp. 1151–1161, by copyright permission of The Rockefeller University Press.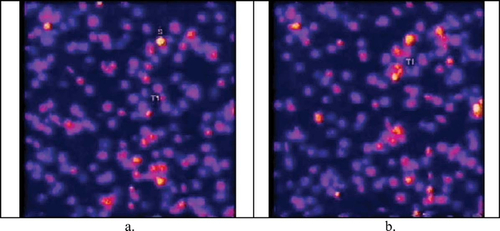
The video is impressive, and it can readily form the basis for a comparison by introductory students of particle velocity in this model with velocities exhibited by intracellular parasites in situ and cells moving by lamellipodia. Moreover, with suitable direction, intermediate students might engage the other experimental results in this paper, specifically those resulting from changes in gelsolin concentration or changes in media viscosity. Do the rates of actin branching and capping, or actin nucleation, limit the resulting motility? Finally, while these data and their explanation provide more insight regarding the cytoplasmic movement of intracellular parasites, it might be instructive for more advanced students to engage in the extensive model building required to orchestrate the fractal nature of these actin-based particle movements into the more complex behavior of lamellipodial locomotion.
Figure 4. The relative movement of 3-, 1-, and 0.5-mm beads in a defined medium favoring the regulated assembly and disassembly of a branching network of actin. The beads show a similar velocity, irrespective of their size, and the actin tail of the large bead in the lower left of each frame continues to grow away from the bead at the same rate, after the bead itself becomes immobilized (at around 8 min). http://www.jcb.org/cgi/content/full/jcb.200207148/DC1/1. Reproduced from The Journal of Cell Biology, 2003, vol. 160, pp. 387–398, by copyright permission of The Rockefeller University Press.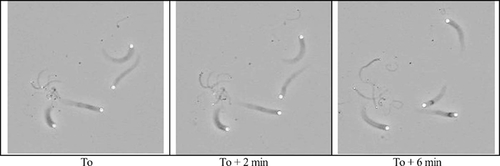
FOOTNOTES
Monitoring Editor: A. Malcolm Campbell